In the intricate world of biopharmaceuticals, suspension cell culture has emerged as a cornerstone technique for viral vector production. As gene therapies and vaccine development gain momentum, the thoughtful integration of suspension culture has become pivotal in addressing the complexities of production and scale-up. This blog post delves into the nuances of suspension culture, unraveling key considerations and strategies essential for successful viral vector production and seamless scale-up processes.
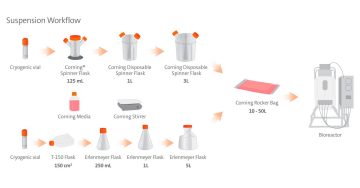
When it comes to developing and scaling up suspension cell culture processes like viral vector production, there are several important factors to consider. In addition to process parameters like media formulation or agitation, it's also important to have more theoretical considerations, like your plans for production at larger scales.
During the development stage, you will have flexibility as you engage in initial exploration and identify your critical process parameters; however, as you move toward manufacturing, certain aspects of your process will become more important, and are worth consideration up front. One of these aspects is your seed train; how will you get from a few million cells to several billion? Inherent to seed train is the scalability of the vessel you use; can you expect similar performance whether you're growing a few hundred milliliters versus several liters? Finally, once you are manufacturing at large scale and in a GMP environment, your process will require closed systems; as such, it's advantageous to familiarize yourself with these systems from an early stage.