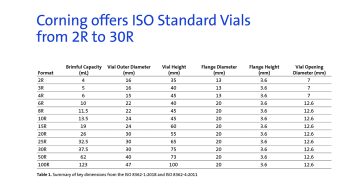
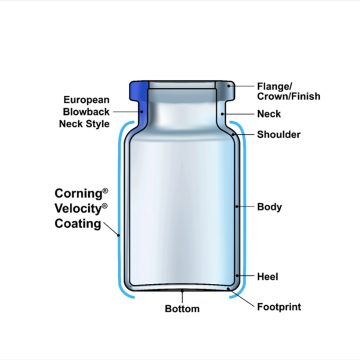
When placing a vial on a flat surface, the bottom refers to the concave face that does not directly contact the surface. The maximum distance between the vial’s bottom and the flat surface, known as the push-up, is specified based on the size of the vial.
The footprint is the part of the vial that does contact the flat surface it sits on. The integrity of a vial’s footprint is essential for the product’s performance in production. If the footprint isn’t uniform the vial can become physically unstable which can increase the likelihood of tip overs and false rejects on the manufacturing line.
The heel is the transitional corner between the footprint and the body of the vial. The radius of the heel can impact the mechanical reliability and machinability of the vial. For example, a smaller radius with a sharp-cornered heel can make a vial more prone to tipping over on the filling line, causing potential cracks and breaks.
The cylindrical mid-section is known as the body of the vial, which should be consistent in diameter to streamline handling on fill-finish lines and inspection equipment. The body of converted tubular vials matches the dimensions of the tubing used.
The shoulder is the section between the body and the neck of the vial. Improper forming of the shoulder might lead to defects that impact machinability. Consistent minor flaring of the shoulder region within a large population of vials can cause damage around the upper circumference of the vial due to glass-on-glass contact in filling lines.
The neck of the vial is the narrow piece between the body and the flange. The dimensions and form of the inner neck are important to ensure consistent stopper insertion and to minimize the likelihood of stopper pop up. The external geometry of the neck is also critical both for handling and consistent performance during the capping process.
The flange of the vial, which is also sometimes referred to as the crown or finish, is one of the most critical aspects. The top of the flange interacts with a compressed elastomer stopper to form what is called the “land-seal” which is primarily responsible for maintaining the sterility of the drug product, also referred to as container closure integrity (CCI).
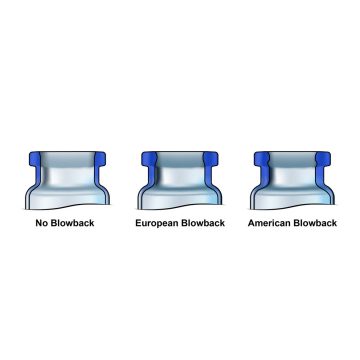
The final part of the vial is known as a blowback, which is only sometimes included in a typical vial to prevent stopper pop up and facilitate the sealing between the stopper and the vial. The European style blowback is a protrusion that creates an undercut while the American style blowback is a groove.