Cell cryopreservation suspends cell line animation, effectively stopping biological time. This may seem like science fiction, but cell cryopreservation is fundamental to cell culturing. It's a meticulous process that requires exquisite precision and care.
Cryopreservation of Cells Step by Step Guide
The cryopreservation of cells requires careful attention. But how do you prepare cells for cryopreservation successfully? Follow these steps:
Step 1: Select the Cells for Cryopreservation
For optimal cryopreservation, cells must be in prime condition and near the end of the logarithmic growth phase. Make sure they have more than 80 percent confluency before freezing and carefully examine cultures for microbial contamination. Using an antibiotic-free medium to grow cultures for several pre-testing passages may foster enough growth to identify otherwise undetectable contaminants.
Examine samples under a microscope, and conduct a direct culture to test for bacteria, fungi, mycoplasmas, and yeasts. Mycoplasmas present unique challenges because they can pass undetected, so make sure to culture stocks again after freezing.

Step 2: Harvest the Cells
Be gentle when harvesting and vary your procedures by cell type. Strive for the optimal freezing concentrations. Low concentrations decrease viability; high concentrations can lead to cell clumping.
Wash off or inactivate any dissociating agents after harvesting because they can damage cells. If you use a centrifuge, only use force hard enough to yield a soft pellet.
Pool the contents of the harvested culture vessels to ensure the uniformity of the final frozen stock. Dilute the concentration of the cell suspension as needed to achieve twice the desired concentration.
Keep cells chilled to slow cell metabolism and prevent clumping. Gas cells with carbon dioxide as necessary to prevent alkaline pH shifts.
Step 3: Store the Cells
Select the right cryoprotective agents, like dimethyl sulfoxide or glycerol, to minimize cell damage. Your storage vessel choice is also critical because improper containers present risks, including injury, vessel damage, contamination, or loss of frozen stock.
The most used cryogenic storage vessels are single-use storage containers, like Corning® cryopreservation bags, and polypropylene screw-capped vials, like Corning internal or external thread cryogenic vials.

Step 4: Cool the Cells for Cryopreservation
Keep your cooling rate steady to ensure cell viability and integrity. It should be slow enough to allow for dehydration but fast enough to prevent dehydration-related damage. For most animal cell cultures, the ideal cooling rate is a steady drop between 1°C and 3°C per minute until they reach -80°C. Larger or less permeable cells might need to cool slower because they dehydrate slower.
Some labs use programmable electronic cooling units to precisely control the freezing process and yield uniform, reproducible results. Other labs use mechanical units that offer sufficient, more affordable process control. Consider using Corning Cool Cell® alcohol-free cell-freezing containers to ensure cells freeze at the ideal cooling rate.
Step 5: Store the Cells
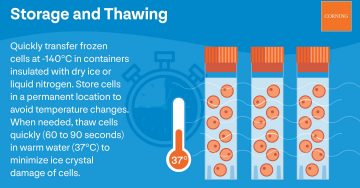
Once cells freeze, it's important to move quickly. Use an insulated container like Corning® CoolCell® for cryopreservation of cells filled with dry ice or liquid nitrogen to transfer the frozen stock to permanent storage. Speed is critical to avoid cell warming or damage.
Most cell culture labs use liquid nitrogen freezers to maintain a consistent temperature below -140°C. Even a brief, temporary temperature rise can damage cells.
Step 6: Thaw the Cells
While you cool cells gradually, you thaw them rapidly. Quick thawing reduces the formation of damage-causing ice crystals inside cells during rehydration.
Put your container in warm water and stir gently until small ice pellets remain. Cells will continue to thaw as you spray them with ethanol and place them in the hood. You will get the best results by thawing cell cultures for 60 to 90 seconds at 37°C.
Step 7: Let the Cells Recover
To avoid damage from prolonged exposure, remove cells from cryoprotective agents quickly and gently. Remove them based on the type of agent and cells. For example, gentle centrifugation can remove agents from cells sensitive to cryoprotective agents.
Be careful when glycerol is used as a cryoprotectant. Do not suddenly add a large volume of fresh medium to thawed cells — this can damage or destroy them. Instead, take the cells through several stepwise dilutions with an equal volume of warm medium every 10 minutes and let them adjust before further processing.
Most cells recover normally if you remove cryoprotective agents through a medium change within 6 to 24 hours of thawing.
Successfully preserved frozen cells need little maintenance. They can be a lifeline if you lose cell cultures to contamination or accidents. Frozen cell cultures can be useful for long-term experiments because their suspended animation ensures limited biological variants.

Cryopreservation Applications
Cryopreservation Applications
Cryopreservation is valuable and beneficial outside of laboratory experiments. It is widely used in:
- Agricultural biotechnology: Cryopreservation is an affordable long-term conservation approach for fruits and vegetables, reducing genetic modification risk.
- Biobanking: Cryopreservation can support the long-term storage of biological samples and cell lines used in biotechnological research and drug development.
- Regenerative medicine: Cryopreservation of stem cells preserves the cells' potential to regenerate damaged tissue and develop new medical treatments.
- Reproductive medicine: Cryopreservation of sperm, egg, ovarian tissue, and ovary cells promotes artificial insemination and in vitro fertilization efforts.
- Tissue engineering: Cryopreservation safeguards engineered tissues and scaffolds used in tissue engineering. The process supports future implantation and potential therapeutic applications.
Cryopreservation Challenges and Troubleshooting
With all its benefits, cryopreservation can still present challenges:
- Ice crystals: Ice crystal formation during freezing is common; however, crystals damage cell structures and membranes, compromising cell viability. Use precise cooling techniques to reduce ice crystal formation.
- Intracellular ice formation: Some cells are vulnerable to intracellular ice formations that reduce cell viability. Excessive intracellular ice formation can lead to dehydration and cell membrane damage. Specialized cryopreservation protocols and methods can protect cells.
- Cryoprotective agents: High concentrations of cryoprotective agents can be toxic, limiting cell functionality and viability. Carefully balance cryoprotective agents to maintain low toxicity for successful cryopreservation.
- Thawing: Improper thawing reduces cell functionality and viability. Use controlled thawing methods to protect cells.
Cryopreservation is integral to a variety of scientific investigations. As its application opportunities expand, understanding the process's intricacies — and challenges — will become even more important.


